Yesterday was a great Monday in Chemistry! We started off class with some demos about elements patterns and properties. Mr. Lieberman brought us over to the flame hood and burned small samples of lithium nitrate (LiNO3), sodium chloride (NaCl), potassium nitrate (KNO3), copper (Cu), and strontium (Sr). Along with admiring the pretty colors that the flames gave off, we also observed that elements in the same group or family tend to have similar properties. We discussed how this might be a real-life application in investigating a crime scene with chemical residue, testing elemental properties, or making fireworks.
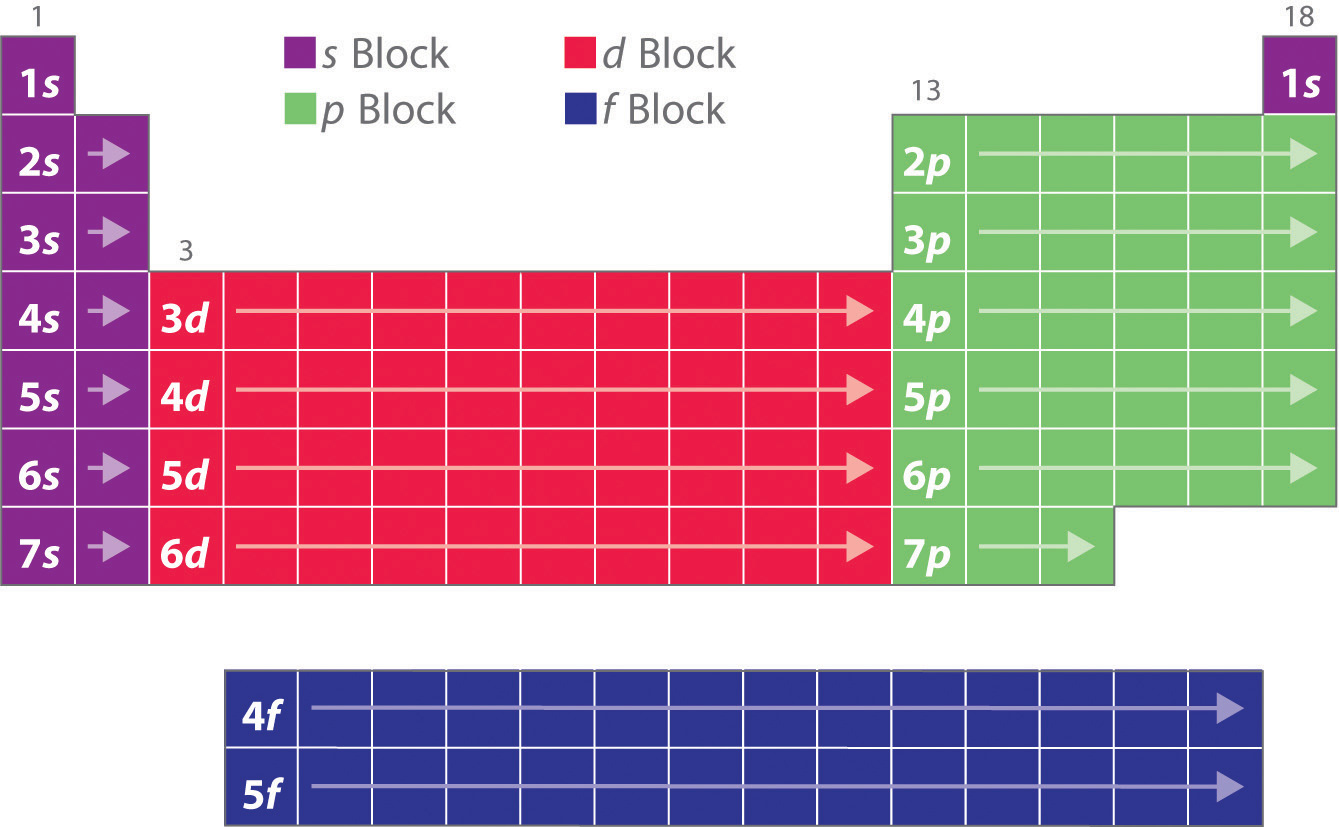
Here's a clear picture of the general patterns we found:
Here's one of every element individually labelled with their ending electron configuration:
(PLEASE NOTE: Helium (He) in the top right is different in that it ends with 1s rather than 1p.)
Towards the end of class, Mr. Lieberman demonstrated how we can use these configurations to abbreviate the entire electron configuration. Since, as good chemists, we only care about the electron that will react, we can use the previous noble gas to speed up the process. For example, if I wanted the electron configuration for uranium (U), I would write [Rn]7s2-5f3 rather than starting all the way from 1s1. If you're confused, make sure to come in for some extra help!
We didn't stamp the homework today, but the rest of the electron configuration packet it due for tomorrow! Also, keep in mind that tomorrow we have a sports assembly during period 2, so if you are missing that for marching band or a sport, make sure to check Moodle for the work you missed!
The next scribe will be... Rachel S.

No comments:
Post a Comment