Today in class we...learned about molar mass!
Here's the basics on molar mass for you Pac Rim people that went to Chinatown today and missed class.
(Please look over the second page of our notes packet if this makes no sense)
Molar mass is just another name for the average atomic mass that can be found on the periodic table (the number usually with decimal places). It tells us the mass of one mole (6.02e23 units) of that substance (on the periodic table, the mass of one mole of that element). Two different substances can be the same amount (one mole) but have different masses.
Because many of the molar masses have multiple decimal places, we round to the nearest whole number when it makes sense to make calculations easier.
E.G.- On the periodic table it says that the molar mass of Carbon is 12.0107, but when using the molar mass to solve a problem, just use 12.
However, for an element like tin (118.710) just round to 118.7
Finding molar mass of compounds (look at "Learning Check!" in notes packet):
Take the compound aspartame as an example. It is C14H18N2O5. To find the total molar mass, find the molar mass of each atom (C, H, N, O) and then multiply it by the subscript.
Answer=294 g
Knowing this will be helpful in word problems like the ones we had on our worksheet homework tonight.
That's the basics of molar mass. It's not that complicated.
Besides learning about this is class, we got to work on our worksheet homework and went over last night's homework. (P.S. we'll go over the quizzes tomorrow in class--they weren't super good :/ )
Don't forget: Avogadro's Number Lab is due tomorrow! (Write out the calculations and the answers, not the procedures).
The next scribe will be Emma B. (have fun!)
A peek inside the everyday happenings of our classroom. This is an interactive learning environment for students and parents in my Honors Chemistry 173 class. This ongoing dialogue is as rich as YOU make it. Visit often and post your comments freely.
Thursday, September 27, 2012
Wednesday, September 26, 2012
Moles
Today In Class:
4. Gathered Data for a mole Lab/Activity
Moles:
A mole is a counting unit used in chemistry. It is like a dozen, ream, etc. Amedeo Avagadro was the scientist who came up with this unit. A mole has a very large quantity, if you had a mole of soft drink cans, it would cover the earth with a depth of over 200 miles! Abbreviated as mol.
Avogadro's Number Conversion Factor:
1 mole = 6.02 x 10^23 particles.
A particle could be an atom or a molecule say pay close attention to your problem!
In order to solve problems with moles we have to use our old friend Dimensional Analysis!!!
Ex. How many moles are in 7.8 x 10^24 atoms of carbon?
7.8 x 10^24 atoms of carbon x 1mole = 13 moles.
1 6.02 x 10^ 23 particles
Homework:
1. Avogadro's Number Worksheet
2. Avogadro's Number Activity (Fill out in the lab book)
The next scribe will be Claire. F.
The next scribe will be Claire. F.
Monday, September 24, 2012
Acids
Today in Class We...
- Got Quiz 2.1 back
- Went over acids and naming them
- Reviewed naming Ionic and Covalent compounds
- Took Quiz over naming Ionic and Covalent compounds

Acids
Acids are substances formed when nonmetal ions combine with hydrogen. The hydrogen is written first in the compound and the naming is based on whether the anion (negative ion) is:- Acids always contain hydrogen.
- Example: Hydrogen and Chlorine:
Naming Acids *Trick to remember Acids: 'ate'ic- 'ite'is
If an anion does not contain oxygen-- change the ending to "ic"
- add prefix hydro
- HF Hydrofluoric acid
If an anion does contain oxygen
- "ate" becomes "ic"
- "ite" becomes "ous"
-HCN- hydrogen + cyanide = hydrocyanic acid
-H2SO3 - anion is sulfite = sulfurous acid
Homework for tonight:
- Naming Workshop worksheet
- Study for naming quiz tomorrow *8 polyatomic ions need to be memorized
Saturday, September 22, 2012
Covalent Bonds
Important Things to Note About Covalent Compounds/Bonds
-Both the first and second elements of the compound are non-metals.
-The second element's name ends with the suffix -ide.
-When naming these compounds, we use Greek prefixes to determine how many atoms of an element are included in the covalent compound. ie. Dinitrogen (2) Pentoxide (5)
-The name of the first element can have any of the different prefixes except for mono-(one).
-The name of the second element can have any of the different prefixes.
Ionic vs. Covalent
-Both the first and second elements of the compound are non-metals.
-The second element's name ends with the suffix -ide.
-When naming these compounds, we use Greek prefixes to determine how many atoms of an element are included in the covalent compound. ie. Dinitrogen (2) Pentoxide (5)
-The name of the first element can have any of the different prefixes except for mono-(one).
-The name of the second element can have any of the different prefixes.
Ionic vs. Covalent
-Ionic compounds consist of one metal element and one non-metal element, but covalent compounds consist of two non-metal elements.
-The elements in an ionic compound must neutralize their charges, but elements in a covalent compound don't need to.
-Elements in ionic compounds don't use prefixes to determine the amount of atoms per element that are in the compound, but elements in covalent compounds use prefixes for their compound names.

Homework
-Covalent Compounds/Bonds Naming Practice worksheet
-Memorize the 8 polyatomic ions for Monday
-Study for the quiz on Monday
Prefixes for the Compound Names

Examples
-Sulfur Pentoxide - The formula would be SO5. The first element is S because we know that sulfur's atomic letter is S. The second element is O5 because we know that oxide is oxygen, and oxygen's atomic letter is O. Then since it has the prefix pent(a)-, we know that there's 5 oxygen atoms so you would put 5 as the subscript.
-Tetraphosphorus Trioxide - The formula would be P4O3. The first element is P4 because we know that phosphorus's atomic letter is P, then it's subscript is 4 because we know that there's 4 phosphorus atoms because of the prefix tetra-. The second element is O3 because we know that oxygen's atomic letter is O, then it's subscript is 3 because we know that there's 3 oxygen atoms because of its prefix tri-.
-You can find other examples in your notes from class.
-Covalent Compounds/Bonds Naming Practice worksheet
-Memorize the 8 polyatomic ions for Monday
-Study for the quiz on Monday
The next scribe will be Courtney S.
Thursday, September 20, 2012
Ionic Bonds
Lesson Overview:
-Ionic bonds are bonds made from sharing electrons which have to be ions of opposite charges
-Learning how to name compounds with metals and nonmetals
-Learning how to name compounds with transitional metals and nonmetals
-A short lesson on polyatomic ions
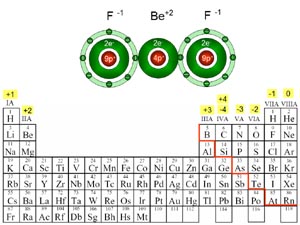
Vocabulary:
Ion- charged particles
Cation- positively charged ion
Anion- negatively charged ion
1)Metals and Nonmetals:
-First element must be metal
-Second must be a nonmetal
-When naming the whole compound, the second element (the nonmetal) must be changed to have an -ide at the end. (e.g. fluoride, oxide, etc.)
-Charges have to cancel out
Examples:
-Naming
NaBr= sodium bromide
CaF2= calcium flouride
BaO= Barium Oxide
-Writing into Compounds
Potassium Chloride= KCl (Potassium has a charge of 1+, and Chlorine has a charge of 1-)
Sodium Sulfide= Na2S (Since sodium has charge of 1+, it needs two to balance with the sulfide which has a charge of 2-)
2)Transitional Metals and Nonmetals:
-First element must be a transition metal
-Second element must be a nonmetal
-Transitional metals can have more than one type of charge, and they are represented by roman numerals. (e.g Cobalt has both a 2+ and 3+ type of charge, so Cobalt (II) and Cobalt (III)- respectively)
-The first must be listed with a roman numeral which represents the charge. It is not a subscript!
-Charges must cancel and the second element still has to end in -ide
Example:
-Naming
FeBr3= Iron (III) Bromide (Since there are three bromide which combine to make a total charge of -3, there has to be be an iron with a charge of +3)
NiS= Nickel (II) Sulfide (Sulfide has a charge of -2, so the nickel also needs to balance out the charges with a charge of +2)
-Writing into Compounds
Copper (I) Chloride= CuCl (Copper has a charge of +1, and chloride has a charge of -1)
Iron (III) Oxide= Fe2O3 (since this iron has a charge of +3 and the oxygen has a charge of -2, there has to be a common multiple. So, there would have to be 2 iron molecules and 3 oxygen molecules)
3) Polyatomic Ions
-combining atoms that carry a charge
-The big eight to memorize
Sulfate= SO4 (charge: 2-)
Hydroxide= OH (charge: 1-)
Ammonium= NH4 (charge: +1)
Nitrate= NO3 (charge: 1-)
Carbonate= CO3 (charge: 2-)
Acetate= C2H3O2 (charge: 1-)
Phosphate= PO4 (charge: 3-)
Bicarbonate= Hydrogen Carbonate= HCO3 (charge 1-)
Examples:
-Formula
Ammonium Sulfate=(NH4)2SO4
Since Ammonium is NH4 with a charge of +1 and sulfate is So4 with a charge of 2-, there has to be be two ammonium ions. To represent two ammonium ions, put the ammonium in parenthesis and write another subscript.
Iron Nitrate= Fe(NO3)3
Iron has a charge of 3+ and nitrate has a charge of -1, so there has to be three nitrate ions to balance out with the 3+ charge of iron.
The next scribe will be: Danielle S.
-Ionic bonds are bonds made from sharing electrons which have to be ions of opposite charges
-Learning how to name compounds with metals and nonmetals
-Learning how to name compounds with transitional metals and nonmetals
-A short lesson on polyatomic ions
Vocabulary:
Ion- charged particles
Cation- positively charged ion
Anion- negatively charged ion
1)Metals and Nonmetals:
-First element must be metal
-Second must be a nonmetal
-When naming the whole compound, the second element (the nonmetal) must be changed to have an -ide at the end. (e.g. fluoride, oxide, etc.)
-Charges have to cancel out
Examples:
-Naming
NaBr= sodium bromide
CaF2= calcium flouride
BaO= Barium Oxide
-Writing into Compounds
Potassium Chloride= KCl (Potassium has a charge of 1+, and Chlorine has a charge of 1-)
Sodium Sulfide= Na2S (Since sodium has charge of 1+, it needs two to balance with the sulfide which has a charge of 2-)
2)Transitional Metals and Nonmetals:
-First element must be a transition metal
-Second element must be a nonmetal
-Transitional metals can have more than one type of charge, and they are represented by roman numerals. (e.g Cobalt has both a 2+ and 3+ type of charge, so Cobalt (II) and Cobalt (III)- respectively)
-The first must be listed with a roman numeral which represents the charge. It is not a subscript!
-Charges must cancel and the second element still has to end in -ide
Example:
-Naming
FeBr3= Iron (III) Bromide (Since there are three bromide which combine to make a total charge of -3, there has to be be an iron with a charge of +3)
NiS= Nickel (II) Sulfide (Sulfide has a charge of -2, so the nickel also needs to balance out the charges with a charge of +2)
-Writing into Compounds
Copper (I) Chloride= CuCl (Copper has a charge of +1, and chloride has a charge of -1)
Iron (III) Oxide= Fe2O3 (since this iron has a charge of +3 and the oxygen has a charge of -2, there has to be a common multiple. So, there would have to be 2 iron molecules and 3 oxygen molecules)
3) Polyatomic Ions
-combining atoms that carry a charge
-The big eight to memorize
Sulfate= SO4 (charge: 2-)
Hydroxide= OH (charge: 1-)
Ammonium= NH4 (charge: +1)
Nitrate= NO3 (charge: 1-)
Carbonate= CO3 (charge: 2-)
Acetate= C2H3O2 (charge: 1-)
Phosphate= PO4 (charge: 3-)
Bicarbonate= Hydrogen Carbonate= HCO3 (charge 1-)
Examples:
-Formula
Ammonium Sulfate=(NH4)2SO4
Since Ammonium is NH4 with a charge of +1 and sulfate is So4 with a charge of 2-, there has to be be two ammonium ions. To represent two ammonium ions, put the ammonium in parenthesis and write another subscript.
Iron Nitrate= Fe(NO3)3
Iron has a charge of 3+ and nitrate has a charge of -1, so there has to be three nitrate ions to balance out with the 3+ charge of iron.
The next scribe will be: Danielle S.
Wednesday, September 19, 2012
September 19th post
Today in class:
-watched a video about the elements : http://www.youtube.com/watch?v=d0zION8xjbM
-went over the notes for protons, neutrons, electrons, atomic symbols, and isotopes (last page of the note packet), and the second box of the first page (the one with the blank periodic table of elements)
-went over some of the lab
Some Notes (check your note packet for more) :
-Protons and neutrons have a relative mass of 1
-Protons determine the atomic number
-Neutron's charge is 0
-Electrons have negative charge and has a relative mass of 0
-Electron is equal to the number of protons in a neutral atom
-Mass number is the number or protons plus the number of neutrons
-Isotopes are two atoms of the same element
Homework:
-Isotopes in Chem Think
-Metals, Non-metals, Metalloids Lab
-Atomic structure worksheet
-Web Assigns (due 10/05)
Next scribe is: Yada Thia
-watched a video about the elements : http://www.youtube.com/watch?v=d0zION8xjbM
-went over the notes for protons, neutrons, electrons, atomic symbols, and isotopes (last page of the note packet), and the second box of the first page (the one with the blank periodic table of elements)
-went over some of the lab
Some Notes (check your note packet for more) :
-Protons and neutrons have a relative mass of 1
-Protons determine the atomic number
-Neutron's charge is 0
-Electrons have negative charge and has a relative mass of 0
-Electron is equal to the number of protons in a neutral atom
-Mass number is the number or protons plus the number of neutrons
-Isotopes are two atoms of the same element
Homework:
-Isotopes in Chem Think
-Metals, Non-metals, Metalloids Lab
-Atomic structure worksheet
-Web Assigns (due 10/05)
Next scribe is: Yada Thia
Tuesday, September 18, 2012
Metal, Non-Metal and Metalliod Lab
By: Katie Hauldren
Today, to kick off the second unit and to start looking at the periodic table, we did the "Metal, non-metal and metalloid lab". Basically we were given 8 unknown elements and we recorded some of their physical and chemical properties. The goal of the lab was to try to figure out what element each unknown was.
(Pictured below is all the unknown elements and two liquids that will help us in the chemical testing)
The physical properties of each element we recorded was their appearance, luster (if it is shiny or dull), malleability (brittle or bendable) and its electrical conduct. We used our eyes (obvi) to conclude their appearance and luster. We used a hammer and cutting board (pictured to left) to test each of their malleability. Finally, we used a electricity conductivity tester to find the levels of electricity (i had a picture but my sister deleted it...)
Then to test the chemical properties of the elements, we wanted to see how the elements would react with HCI and CuCI2. We used the plastic dish (photographed to the right) to carry out this part of the expirament. It had a "chemical reaction" if the element bubbled, changed color, changed temperature, in other words if the chemical didn't stay the same.
Today was a GR8 lab and I hope everyone learned a little more about some elements in the periodic table.
REMEMBER: Mr. Lieberman only put 7 elements on the back side of your lab so you are going to have to do some research to figure out the last one.
Homework: Do lab (Don't fret, its not due until Thursday.) and Web Assign 2.3 is up!
peace love chemistry
Next scribe is... Stacie Cho
Labels:
Katie H,
Lab,
Metal Non-metal metalloid lab,
Unit 2
Subscribe to:
Posts (Atom)



